Search
Democracy Links
Member's Off-site Blogs
hey wayne - yet again .....
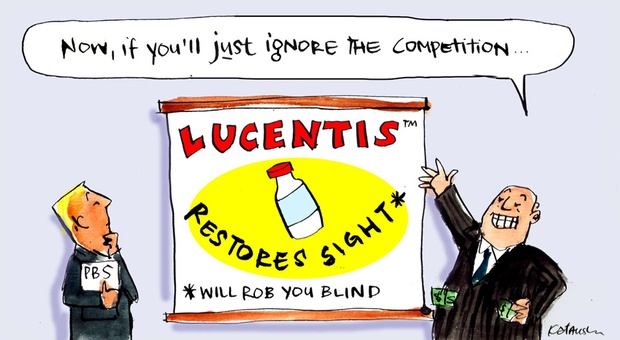
What if the government could save hundreds of millions of dollars by listing a cheaper drug, shown to be just as effective as the one which costs the PBS more than any other medicine? You won’t find out so long as the company that owns both drugs keeps the bargain-priced cousin shut out.
Just on a year ago, the United States National Institutes of Health put out a press release under the headline: “Avastin and Lucentis are equivalent in treating age-related macular degeneration.”
So what, you say?
So this: America’s - and one of the world’s - foremost medical research agencies had not just pronounced judgement on the relative clinical benefits of two drugs for treating the most common cause of blindness in the developed world.
In fact, it had come down decisively on one side of one of the hot medical/legal issues, and arguably one of the great drug-industry cons, of recent times. Over the past six or seven years, several related drug companies have extracted billions of dollars from health systems around the world by charging roughly 40 times as much as was necessary to treat macular degeneration.
So even if you have perfect vision this concerns you. Wherever you are, someone is footing the medical bills; in Australia, hundreds of millions of your tax dollars are at stake.
For while the two drugs, developed by the same company, have been determined in some major studies to be clinically equal, they are not equal in price. The cost of Avastin, in the United States as here in Australia, is about $50 per treatment. The cost of Lucentis is around $2,000 per treatment.
Unsurprisingly, the drugs’ makers have done all they can to encourage the use of the expensive one and to frighten doctors and patients away from the cheap one, as you will soon see. Equally unsurprisingly, this has provoked conflict around the world, with doctors and governments concerned about getting value for the health dollar.
But at the end of a comprehensive, independent, two-year study, the so-called CATT study (for Comparison of Age-related macular degeneration Treatment Trials), supported by the US National Eye Institute, the two were found to be equally “safe and effective therapies”.
The Lucentis/Avastin story serves an object lesson in the lengths big pharma companies will go, to maximise their profits. It starts in the US, but it runs around the world, and there is a $300 million-a-year sting in the tale for Australia.
Let’s go back to the beginning. And in the beginning was Genentech, a company founded in 1976 and generally considered to have started the biotechnology medical revolution.
In 2009, Genentech was taken over by the Swiss pharma giant, Roche. Roche paid a big price, almost US $47 billion, and initially there were widespread concerns about how the culture of Genentech - innovative and informal, a lot like the tech companies of Silicon Valley - would mesh with that of Roche.
But the deal paid off, big time, for Roche. As noted by Reuters’ Caroline Copley in a 2012 analysis: “Three years on ... the jeans-wearing scientists in San Francisco have proven they are the ones driving the drugs pipeline of the 116-year-old Basel-based pharma giant, casting a shadow over Roche’s own research operations.
“Roche’s four top-selling medicines in 2011 - Rituxan, Avastin, Herceptin and Lucentis - were all cooked up in Genentech’s labs and accounted for 55 per cent of total pharma sales last year.”
There’s one more corporate connection you need to understand: another Swiss pharma giant, Novartis, owns one-third of Roche, and is responsible for marketing the expensive drug, Lucentis, outside the United States.
Now, about those two top-selling drugs, Avastin and Lucentis.
- By John Richardson at 11 May 2013 - 5:49pm
- John Richardson's blog
- Login or register to post comments
Recent comments
2 hours 2 min ago
11 hours 43 min ago
12 hours 31 min ago
19 hours 32 min ago
23 hours 11 min ago
1 day 1 hour ago
1 day 1 hour ago
1 day 2 hours ago
1 day 2 hours ago
1 day 10 hours ago